Specific heat of a gas is same in both isochoric and isobaric process
Physics
DOI:
https://doi.org/10.14331/ijfps.2018.330116Keywords:
Specific Heat, Isothermal, AdiabaticAbstract
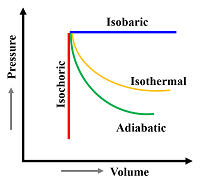
The Palchoudhury gas theory and Palchoudhury gas equation have a profound potency applicable to all phenomena of gases. Inner and covered outer surface area of molecules are the outstanding inventions related to gas behaviour. The exertion of infrared wave force on the covered outer surface area of tiny particles and the corresponding effect on the inner surface of a gas container is the real cause of the gas behaviour. We can independently explain all kind of behaviour of gases with the help of the conception the inner force and internal force. We can clarify about the isothermal and adiabatic behaviour of gases, show the relation between heat and force. It is most significant finding, the specific heat of an identical gas in the isochoric and the isobaric process is not different but same as the inner force in both methods is same.
Downloads
References
Palchoudhury, S. (2016). A Modification in Behaviour of Gases. International Journal of Fundamental Physical Sciences (IJFPS), 6(3), 4.
Palchoudhury, S. (2017a). About Internal Force of Gases. International Journal of Fundamental Physical Sciences, 7(4), 34-37.
Palchoudhury, S. (2017b). Brownian Motion and Infrared Wave Force. International Journal of Fundamental Physical Sciences(IJFPS), 7(2), 23-24.
Published
How to Cite
Issue
Section
License
Copyright (c) 2018 International Journal of Fundamental Physical Science

This work is licensed under a Creative Commons Attribution 4.0 International License.


 https://orcid.org/0000-0001-6275-8624
https://orcid.org/0000-0001-6275-8624








