The effect of reflux process on the size and uniformity of FePt nanoparticles
FePt Nanoparticles
DOI:
https://doi.org/10.14331/ijfps.2012.330014Keywords:
Chemical Synthesis, Reflux, FePt Nanoparticles, UniformityAbstract
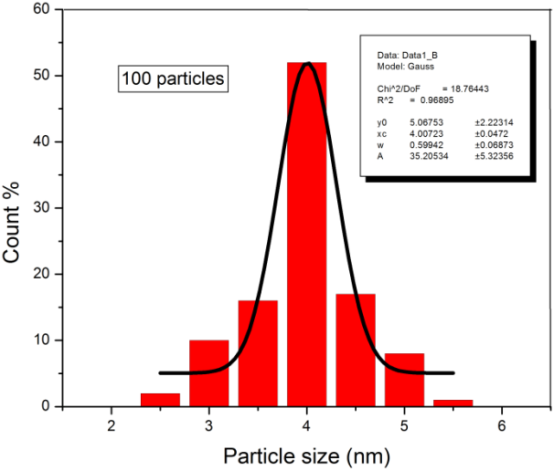
FePt nanoparticles attract great research interest for possible application to ultrahigh density magnetic recording media. In this paper, FePt magnetic nanoparticles were synthesized by superhydride reduction of FeCl2 and Pt(acac)2 at high temperature. Adding superhydride (LiBEt3H) to the phenyl ether solution of FeCl2 and Pt(acac)2 in the presence of oleic acid, oleylamine, and 1,2-hexadecanediol at 200 °C, followed by refluxing at 250 °C, led to monodisperse 3 nm FePt nanoparticles. The effect of reflux process on the size and uniformity of FePt nanoparticles has been investigated. TEM images showed that the size of FePt nanoparticles increase to 4 nm with reflux process and the standard deviation of FePt nanoparticles increase to 10 % which lead to improve the uniformity of FePt nanoparticles. The results of EDS analysis showed that the Fe shell around FePt nanoparticles increase with increasing reflux time and the composition of FePt nanoparticles gives Fe68Pt32 after 10 min reflux.
Downloads
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2011 International Journal of Fundamental Physical Science

This work is licensed under a Creative Commons Attribution 4.0 International License.










