Extraction Pb(II) by (Z)-Furan-2-carbaldehyde Thiosemicarbazone adsorbed on surfactant coated alumina before determination by FAAS
Determination of lead
DOI:
https://doi.org/10.14331/ijfps.2011.330019Keywords:
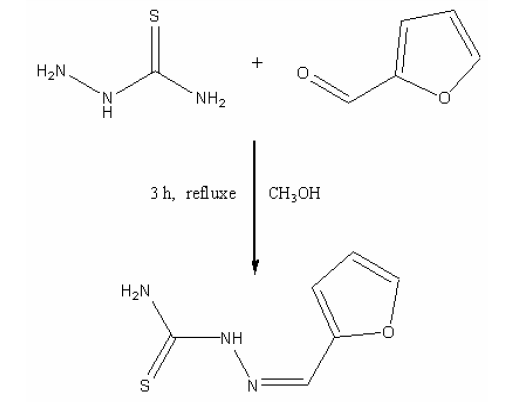
Determination of lead, Pre-concentration, (Z)-Furan-2-carbaldehyde Thiosemicarbazone (I), FAASAbstract
A simple, highly sensitive, accurate and selective method for determination of trace amounts of Pb(II) in water samples is presented. The method is based on selective chelation of Pb(II) on surfactant coated alumina, modified with a Schiff’s base (Z)-Furan-2-carbaldehyde Thiosemicarbazone (I). The retained ions were then eluted with 4 ml of 4 M nitric acid and determined by flame atomic absorption spectrometry(FAAS) at 283.3 for Pb. The influence of flow rates of sample and eluent solutions, pH, breakthrough volume, effect of foreign ions on chelation and recovery were investigated. 1.5 g of surfactant coated alumina adsorbs 40 mg of the Schiff’s base which in turn can retain 15.0±0.9mg of each of the two ions. The limit of detection (3σ) for Pb(II) was found to be 5.57 ng l -1. The enrichment factor for both ions is 100. The mentioned method was successfully applied on determination of lead in different water samples. The ions were also speciated by means of three columns system.
Downloads
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2011 International Journal of Fundamental Physical Science

This work is licensed under a Creative Commons Attribution 4.0 International License.










